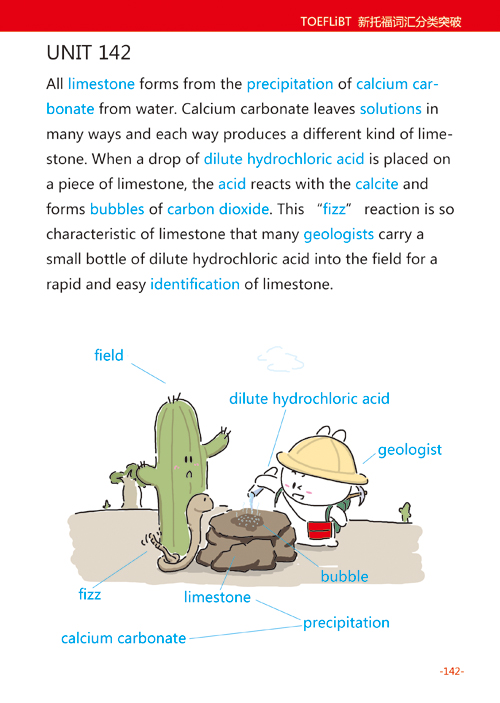
All limestone forms from the precipitation of calcium carbonate from water. Calcium carbonate leaves solutions in many ways and each way produces a different kind of limestone. When a drop of dilute hydrochloric acid is placed on a piece of limestone, the acid reacts with the calcite and forms bubbles of carbon dioxide. This "fizz" reaction is so characteristic of limestone that many geologists carry a small bottle of dilute hydrochloric acid into the field for a rapid and easy identification of limestone.
- limestone [ˈlaimˌstəun] n.石灰岩
- precipitation [priˌsipiˈteiʃən] n. 沉淀
- calcium carbonate [ˈkælsiəm - ˈkɑ:bəneit] n.碳酸钙
- solution [səˈlu:ʃən] n. 溶液
- dilute [daiˈlju:t] adj.稀释的
- hydrochloric acid [,haɪdrəˈklɔ:rɪk - ˈæsid] n.盐酸
- acid [ˈæsid] n. 酸
- calcite [ˈkælsait] n.方解石
- bubble [ˈbʌbl] n. 气泡
- carbon dioxide [ˈkɑ:bən - daɪˈɔksaɪd] n.二氧化碳
- fizz [fiz] n. 嘶嘶声
- geologist [dʒiˈɔlədʒist] n.地质学家
- field [fi:ld] n. 野外
- identification [aiˌdentifiˈkeiʃən] n.鉴定